Bernadette Holdener, PhD
Research interests overview:
My lab is interested in understanding why normal development of the brain, lung, skeletal,
and cardiovascular system require rare forms of oxygen-linked (O-) glycosylation.
These unusual sugars modifications are critical for the function of a limited number
of proteins with thrombospondin type I repeats (TSRs, O-linked glucose-fucose disaccharide)
and epidermal growth factor motifs (EGFs, O-linked glucose). For our studies, we utilize
mouse knockout or conditional knockout mutations to block the addition of these sugars
to the protein modules.
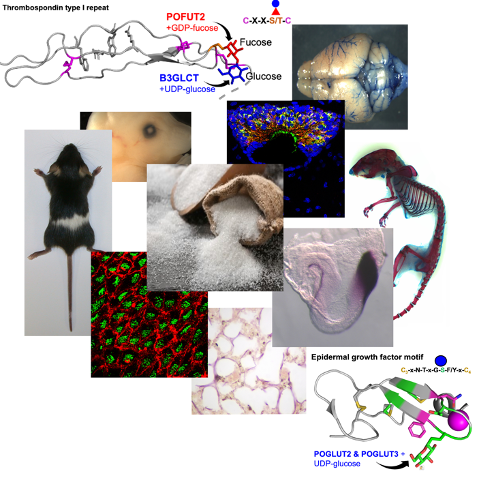
The mutant mice provide us with a better understanding of the molecular mechanisms that contribute to development of chondrodysplasias, hydrocephalus, bronchopulmonary dysplasia, and Marfan’s disease, and identify this subset of glycoproteins in modulating the composition of the extracellular matrix and bioavailability of key cell signaling molecules.
Blocking addition of O-linked glucose-fucose to TSRs alters extracellular matrix composition and cell-signaling in developing bone and brain: Marked shortening of the bones occurs if the glucose-fucose disaccharide is not added to TSRs (Pofut2, Protein O-fucosyltransferase conditional mutant) or the fucose is not extended to the disaccharide (B3glct, Beta-3-glucosyltransferase mutant). In Pofut2 conditional mutants, chondrocyte development is delayed because of an altered fibrillin microfibril network with consequent changes in TGFb and BMP signaling. In B3glct mutants, preventing the elongation of fucose to the disaccharide also leads to congenital hydrocephalus resulting in part from altered polarity of the ependymal cells and likely aggregation of proteins in the central aqueduct. The primary effect of these mutations is likely on the ADAMTS/TSL family of secreted metalloproteinases and related proteins, and current studies focus on understanding whether loss of the O-linked disaccharide on ADAMTS/TSL family members alters their activity and/or extracellular matrix interactions.
Blocking addition of O-linked glucose to EGFs alters fibrillin microfibril and elastic fibril networks in developing lung airway, alveoli, and blood vessels (unpublished): Protein O-glucosyltransferase 2 and 3 (POGLUT2 & 3) were recently shown to add O-linked glucose on EGFs of Fibrillin and Latent TGFb proteins. We are evaluating the effects of the Poglut2/3 double knockouts (DKOs) on cardiopulmonary development. The DKOs neonates are runted and die soon after birth from cardiopulmonary defects. Current studies focus on evaluating the impact of the Poglut2/3 DKO on the assembly and physical properties of the fibrillin microfibrils and the determining the consequence of these changes on cell-signaling and cell differentiation during airway branching and alveolarization and cardiovascular development.
Bernadette Holdener, PhD, Professor
Department of Biochemistry and Cell Biology
Bernadette.holdener@stonybrook.edu
Biochemistry & Cell Biology Page